Mouse Elevated Cross Maze Market Accelerates Globally with Strong Growth in APAC, Europe, USA, and Saudi Arabia
Global mouse elevated cross maze market grows with rising neuroscience research demand across APAC, Europe, USA, and Saudi Arabia.
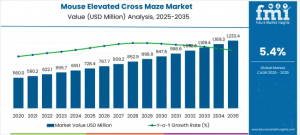
ITALY, November 12, 2025 /EINPresswire.com/ -- The Mouse Elevated Cross Maze Market is entering a period of steady expansion, projected to grow from USD 728.4 million in 2025 to USD 1,232.4 million by 2035, registering a CAGR of 5.4%. Growth is particularly strong across Asia Pacific, Europe, the United States, and Saudi Arabia, where investment in behavioral science infrastructure and preclinical drug development continues to accelerate. This apparatus remains indispensable for evaluating anxiety, cognitive patterns, and exploratory behavior in rodents, forming a critical foundation for neuropharmacology and psychiatric drug discovery.
Explore trends before investing – request a sample report today!
https://www.futuremarketinsights.com/reports/sample/rep-gb-26633
Increasing Clinical and Academic Adoption in Key Regions
Driven by rising demand for standardized behavioral assessment tools, North America and Europe continue to maintain leadership due to their established research ecosystems. However, Asia Pacific—especially China and India—is rapidly closing the gap with aggressive research infrastructure expansion and increased government funding for neuroscience and pharmaceutical research. Saudi Arabia and broader GCC markets are also investing strategically to expand biomedical research capabilities as part of national science and innovation goals.
Academic institutions account for the largest share of usage, representing approximately 42% of total market demand. Pharmaceutical drug discovery applications follow with 28% share, particularly in antianxiety drug development where standardized behavioral metrics support regulatory submissions.
Technology Advancements Supporting Data Precision and Reproducibility
Market growth is reinforced by significant improvements in automated video tracking, high-resolution behavioral scoring, and AI-driven analytics. These technological upgrades reduce manual scoring errors while enabling more detailed and reproducible classification of anxiety-related behaviors. Manufacturers are introducing modular maze platforms that support configurable layouts, multi-parameter tracking, and integration with laboratory information management systems.
Leading participants in this space include San Diego Instruments, Stoelting, Med Associates Inc., CleverSys, and Ugo Basile. Their focus on sensor accuracy, user-friendly software, and compatibility with a broad range of experimental protocols is shifting user preference toward premium systems.
Strong Growth Prospects Across APAC, Europe, USA, and Saudi Arabia
Asia Pacific is projected to lead market expansion, driven by a surge in neuropharmacology and behavioral toxicology research. China is expected to grow at a 7.3% CAGR, supported by government-backed neuroscience initiatives. India follows at 6.8%, reflecting increasing partnerships between universities and pharmaceutical developers.
Europe maintains significant demand, especially in Germany, the UK, and France, where advanced behavioral research clusters continue to scale. In the United States, well-established neuroscience and pharmaceutical research facilities ensure continued market stability. Meanwhile, Saudi Arabia is prioritizing biomedical research modernization, driving selective but high-impact equipment adoption across institutional laboratories.
Application Outlook and Research Drivers
The antianxiety drug development segment represents the highest demand share (approximately 59%), due to increasing global focus on mental health disorders and the growing development pipeline for anxiolytic therapies. Additional use cases include stress-response modeling, cognitive behavior studies, neurodegenerative disease research, and toxicological safety testing.
Demand is further strengthened by the need for standardized protocols that allow cross-laboratory comparability and enhance regulatory confidence in preclinical study outcomes.
Buy Report Now – Click Here to Purchase the Report:
https://www.futuremarketinsights.com/checkout/26633
Latest Medical Supplies Reports:
Newborn Identification Tag Market
https://www.futuremarketinsights.com/reports/newborn-identification-tag-market
Rotary Shaping File Market
https://www.futuremarketinsights.com/reports/rotary-shaping-file-market
Pressure Relief Dressing Market
https://www.futuremarketinsights.com/reports/pressure-relief-dressing-market
Subscribe for Year-Round Insights → Stay ahead with quarterly and annual data updates:- https://www.futuremarketinsights.com/reports/brochure/rep-gb-26633
Why Choose FMI Empowering Decisions that Drive Real-World Outcomes:- https://www.futuremarketinsights.com/why-fmi
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analystsworldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.